The National Comprehensive Cancer Network (NCCN) provides guidelines that outline how to best screen for, prevent, and treat cancer, including determining who should be offered genetic testing for hereditary cancer risk and how individuals should be followed after testing. We’ve summarized some of the recent guideline updates below. The full Genetic Breast/Ovarian guidelines can be found here, and the full Genetic Colon guidelines can be found here. We’ve previously published a list of risk factors that, if present in your personal or family history, should prompt consideration of seeing a certified genetic counselor.
HERE ARE THE UPDATES:
A. TERMINOLOGY
- The NCCN now uses “pathogenic or likely pathogenic variant” instead of the term “mutation” when referring to a difference in a gene that is believed to be harmful. Medically, “pathogenic” and “likely pathogenic” variants are usually treated the same, and are assumed to increase risk for specific conditions.
B. I HAVE A PATHOGENIC BRCA VARIANT. HOW SHOULD I BE FOLLOWED?
- Women with a pathogenic BRCA2variant who are planning to undergo risk-reducing salpingo-oophrectomy (removal of their ovaries and fallopian tubes) may consider delaying surgery until age 40-45, especially if they have already undergone risk-reducing mastectomy. However, special attention should be given to the ages of ovarian cancer diagnoses in your family when making these decisions. For example, a close relative diagnosed with ovarian cancer in her 40s or younger would warrant consideration of surgery at an earlier age.
- The potential risk of uterine cancer in women with a pathogenic BRCA variant has been researched and debated for many years. New, limited data add to the research that suggests there may be a slightly increased risk of a particular type of uterine cancer called serous uterine cancer in women with a pathogenic/likely pathogenic BRCA1variant. Whether this link exists, how high the risk of this type of uterine cancer is, and how important this potentially small increased risk is must be studied further. It is important that you discuss the pros and cons of having your uterus removed at the same time as risk-reducing salpingo-oophorectomy (RRSO) with your health care provider.
C. LYNCH SYNDROME.
- There are multiple computer models that use personal and family history and help identify who should be offered Lynch syndrome testing. Recent data suggest that genetic testing may be appropriate for people with a 2.5% risk to test positive for Lynch syndrome.
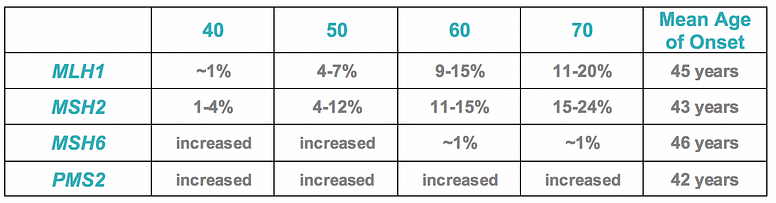
- Age-specific ovarian cancer risks were outlined and the table is adapted below:
- The NCCN panel concluded that there are not enough data to support screening for gastric, duodenal, and more distal small bowel cancer. However, if considered, screening may include upper endoscopy with visualization of the duodenum at the time of colonoscopy every 3–5 years beginning at age 40 (or earlier, based on family history of disease). Testing and treating H. pylori could also be considered.
- Some studies suggest that people with Lynch syndrome may be at risk for pancreatic, breast, and prostate cancers. The panel is not able to make specific screening recommendations for these cancers because of limited data.
D. ANY OTHER UPDATES?
- The guidelines for breast cancer screening and risk reduction for individuals with Li-Fraumeni (pathogenic TP53 variants) and Cowden syndrome (pathogenic PTEN variants) have included the option of risk-reducing mastectomy (RRM) to reduce the risk of breast cancer. The updates suggest that discussion of this surgery should also include level of risk reduction provided by RRM, reconstruction options, age-related cancer risks, family history, and overall health.
- For men and women with Li-Fraumeni syndrome (pathogenic TP53 variants), yearly whole-body MRI has been suggested as a screening option. The updated NCCN guidelines note that while this screening may be beneficial in that it could lead to the early detection of cancer, it may also result in false-positive findings and the identification of tumors that may never cause symptoms.
- For women with Cowden syndrome (pathogenic PTEN variants), further details on recommendations for uterine cancer screening were outlined, including the importance of education and awareness of uterine cancer symptoms, and reiterating that screening for uterine cancer has limitations but that endometrial biopsy every 1-2 years may be considered.
- Information on ovarian cancer risk and management for women with a pathogenic ATM variant was revised from “no increased risk” to “potential increase in ovarian cancer risk, with insufficient evidence for recommendation of risk-reducing salpingo- oophorectomy.”
- Breast cancer risk and management for women with a pathogenic BRIP1 variant was revised from “no increased risk of breast cancer” to “unknown or insufficient evidence.”
- BARD1 was added as a gene that may be linked with increased breast cancer risk, but data are too limited to make specific breast cancer risk management recommendations for women with a pathogenic BARD1 variant at this time. The risk of ovarian cancer is described as “unknown or insufficient evidence”.
Other updates can be found in full documents referenced below.
NCCN Genetic/Familial High-Risk Assessment: Breast and Ovarian Version 1.2019
Genetic/Familial High-Risk Assessment: Colorectal 1.2018
NCCN Genetic/Familial High-Risk Assessment: Breast and Ovarian Version 1.2018
NCCN Clinical Practice Guidelines in Oncology: Breast Cancer Version 4.2017

